A.T. Salminen, D.J. Austin, E. Rodgers, J.A. Willoughby, Sr., J.M. McKim, Jr.
LifeNet Health, Life Sciences, The Institute of Regenerative Medicine, LLC, Virginia Beach, VA.
Abstract
Background and Purpose: Dysfunctional thyroid hormone production or maintenance is implicated in abnormal organ growth,
development, and energy metabolism. Reduction in thyroxine (T4) levels in fetal and neonatal development specifically can result in
severe adverse effects on brain and skeletal development. Thus, evaluating chemical inhibition of T4 synthesis is a key step in the risk
assessment of new chemical entities with direct effects on the thyroid and their potential for endocrine disruption. New approach
methodologies (NAMs) are increasingly being used to test for endocrine disrupting compounds (EDCs), offering a more humanrelevant
alternative to traditional animal testing.
Methods: Human thyroid microtissues were cultured as follows: primary human thyrocytes were isolated from donated human tissue,
made possible by the generous gift of an individual or their family in the LifeNet Health tissue procurement network. Thyrocytes were
plated on Matrigel-coated 96-well plates at a seeding density of 8,000 cells per well in human thyrocyte plating medium (LifeNet
Health). After 48h in culture, media was exchanged with fresh human thyrocyte plating medium supplemented with bovine thyroid
stimulating hormone (TSH), and media was exchanged every other day thereafter. A non-TSH treated control culture was prepared in
parallel for comparison. On day 10 of culture, microtissues were exposed to known EDCs at three concentrations in culture medium,
bracketing half-maximal inhibitory concentrations (IC50) reported in the literature. Media with EDCs was refreshed on day 12 of
culture and collected 48h later (day 14 of culture) for analysis. Media T4 levels were quantified by ELISA (Thyroxine (T4) Competitive
ELISA Kit, ThermoFisher) on the day of collection. IC50 values were determined by a four-parameter least squares fit of the dose
versus response (T4) curve in GraphPad Prism 10. Human thyroid microtissue health was additionally assessed via the CellTiter-Glo®
Luminescent Cell Viability Assay Kit (Promega).
Results: Primary human thyrocytes cultured on Matrigel formed microtissues with uniform size (~80 μm in diameter) and distribution.
Human thyroid microtissues stimulated with TSH showed a 1.85-fold increase in mean 48h T4 production (4.64 ± 0.95 ng/mL) in the
media as compared to cultures without TSH (2.51 ± 1.17 ng/mL). In either case, T4 production was substantially increased in the
microtissue model as compared to primary human thyrocytes cultured in a monolayer (no Matrigel; 0.246 ng/mL). Ten known EDCs
with direct inhibitory activity on thyroid peroxidase (TPO) resulting in reduced T4 production were evaluated in the microtissue system
and their IC50 values for the inhibition of T4 synthesis were determined. Eight IC50s were able to be calculated: catechin (IC50 =
18.64 μM), kaempferol (IC50 = 0.17 μM), methimazole (IC50 = 0.08 μM), resorcinol (IC50 = 0.63 μM), triclosan (IC50 = 24.23 μM),
2,2,4,4-tetrahydroxybenzophenone (IC50 = 7.82 μM), epigallocatechin gallate (IC50 = 42.08 μM), and genistein (IC50 = 14.51 μM).
Sinapinic acid and 6-propyl-2-thiouracil also demonstrated a dose-dependent decrease in T4 production, but did not permit a stable
least squares fit at the exposure concentrations and times tested. Triclosan showed significant cytotoxicity (microtissue culture
reduction in ATP) compared to vehicle at concentrations near the IC50 for T4 inhibition. These data highlight the importance of
monitoring cell viability to differentiate primarily cytotoxic from thyroid disrupting compounds.
Conclusions: The human thyroid microtissue platform, paired with the T4 ELISA and CellTiter-Glo® Cell Viability Assay, is a
promising NAM for identifying new chemical entities with potential for thyroid disruption, early in the product development cascade.
These data support a key molecular initiating event (inhibition of TPO) in the thyroid adverse outcome pathway, enhancing next
generation risk assessment (NGRA).
Introduction
Chemicals that disrupt thyroid function can cause pronounced effects on thyroid hormone (TH) homeostasis resulting in significant
adverse effects in humans. Thyroid hormone production in vivo is controlled through the hypothalamus-pituitary-thyroid axis (HPT).
Perturbations in systemic TH levels can occur when one or more pathways involved in synthesis or degradation are impacted. There
are currently few, if any, human primary thyrocyte models that possesses all phases of TH synthesis, and the use of animals for
toxicity testing is time consuming and expensive. Here, we describe a fully human in vitro 3D microtissue system that retains the
capacity to synthesize and secrete T4 through activation of the TSH receptor, with sufficient dynamic range and stability to enable
detection of perturbations over a reasonable chemical exposure window. The model was challenged with ten endocrine disrupting
compounds (EDCs) known to have direct effects on thyroid peroxidase (TPO), one of the molecular initiating events (MIEs) in the
adverse outcome pathway (AOP). Inhibition of TPO results in reduced production of T4. Observed effects were compared to the
known effects described in the literature.
Materials & Methods
Primary human thyrocytes were isolated from donated human tissue, made possible by the generous gift of an individual or their family
in the LifeNet Health tissue procurement network. For the initial assays to determine T4 production, thyrocytes were prepared in
human thyrocyte plating medium (LifeNet Health) and were either plated in standard cell culture treated 96-well plates (8,000
cells/well) or on a base layer of Matrigel (Corning) in 96-well plates (8,000 cells/well). For all subsequent assays, the thyrocytes were
prepared in human thyrocyte plating medium (LifeNet Health) and plated on a base layer of Matrigel at 8,000 cells per well, in 96-well
plates. After 48h in culture, media was exchanged with fresh human thyrocyte plating medium supplemented with bovine TSH (Sigma),
and media was exchanged every other day thereafter. On day 9 of culture, TSH was refreshed and microtissues were exposed to
known thyroid disrupting chemicals at three concentrations. The exposures (with TSH) were refreshed on day 1, day 3, and day 5 postinitial
exposure. After 6 days (144 hours) of TSH stimulation and compound exposure (15 total days in culture), T4 production and cell
viability was assessed.
Compounds selected were known thyroid disrupters, known to inhibit T4 production. Stock concentrations were prepared in dimethyl
sulfoxide (DMSO; Sigma) at 1000x concentrations and diluted in thyrocyte plating media, resulting in 0.1% final DMSO concentration in
all exposures. Catechin, Genistein, Kaempferol, and Sinapinic acid were from Cayman Chemical. Methimazole, Resorcinol, Triclosan,
2,2,4,4-Tetrahydroxybenzophenone, Epigallocatechin gallate, and 6-Propyl-2-thiouracil were from Sigma.
Thyroxine (T4) production was analyzed in culture media using the Thyroxine (T4) Competitive ELISA Kit (ThermoFisher). Cell viability
was determined by assessing intracellular ATP using CellTiter-Glo® Cell Viability Assay (Promega).
Results
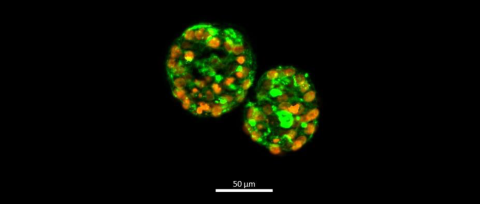
Fig. 1 Fluorescent imaging of human thyroid microtissues showing the follicle-like morphology of microtissues. Microtissues were cultured as described and stained with DAPI (orange) and phalloidin (green) on day 9. Magnification ×100. Scale bar = 50 μm.
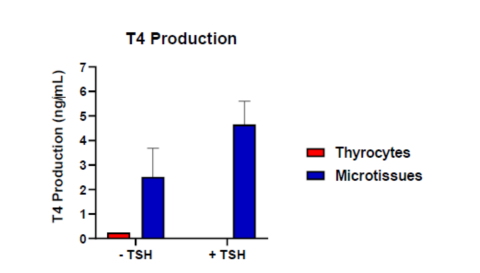
with and without TSH Stimulation.
Fig. 2. Human Thyroid Microtissues were cultured on Matrigel as described. Thyrocytes were also plated on standard cell culture treated 96-well plates in the absence of Matrigel. After 48 hours, cultures were stimulated with TSH, and T4 production (ng/mL) was analyzed in the culture media by ELISA.
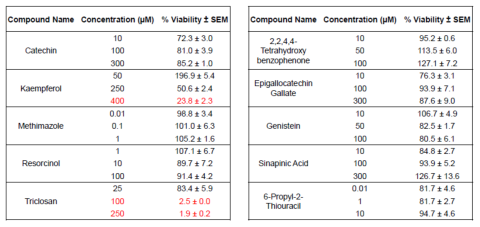
Table 1. Viability of Human Thyroid Microtissues after 144 hours of exposure to 10 chemicals known to disrupt T4 production in vivo (thyroid disrupting chemicals). The concentrations used bracket the known IC50 of these chemicals in vivo. Red lettering indicates % viability < 50% of vehicle control.
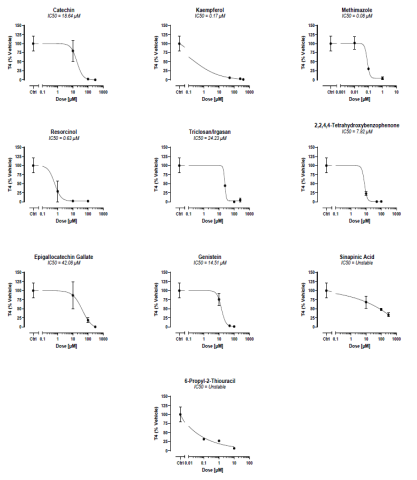
Fig. 3. Human thyroid microtissues were induced with bovine TSH to produce T4 and challenged by the 10 EDCs for 6 days. Culture media was assessed for inhibition of T4 production versus the vehicle control. T4 was analyzed using the Thyroxine (T4) Competitive ELISA Kit from ThermoFisher and data is expressed as total % of T4 produced versus vehicle control. The IC50 was calculated using GraphPad Prism.
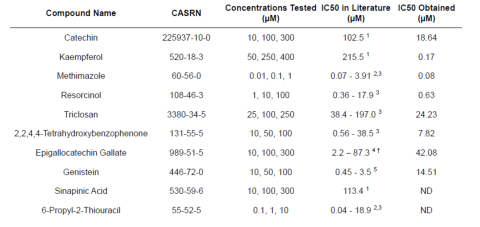
Table 2. The IC50s for inhibition of T4 production of each EDC was determined using GraphPad prism and compared to values identified in the literature. ND = no data. Superscript numbers indicate the referenced literature where the IC50 values were identified. † = IC50s of various receptor tyrosine kinases.
Conclusions
- When plated on Matrigel, primary human thyroid cells form stable 3D human thyroid microtissues with robust T4 production upon TSH stimulation.
- Compound cytotoxicity is an important endpoint when evaluating TPO inhibition.
- At relevant in vitro doses, all ten direct TPO inhibitors reduced production of T4 at concentrations consistent with those reported in the literature.
- TPO inhibition is one of the MIEs in the thyroid AOP. Therefore, the Human Thyroid Microtissues are a promising NAM for identifying new chemical entities with potential for thyroid disruption.
References/Acknowledgements
- Habza-Kowalska, E.; Gawlik-Dziki, U.; Dziki, D. Mechanism of Action and Interactions Between Thyroid Peroxidase and Lipoxygenase Inhibitors Derived from Plant Sources. Biomol. 2019, 9, 663-678.
- Rogers, E.; Breathwaite, E.K.; Nguyen-Jones, T.; Anderson, S.M.; Odanga, J.J.; Parks, D.T.; Wolf, K.K.; Stone, T.; Balbuena, P.; Chen, J.; Presnell, S.C.; Weaver, J.R.; LeCluyse, E.L. Characterization of a Human Thyroid Microtissue Model for Testing Thyroid Disrupting Chemicals. Front. Toxicol. 2024, 6:1408808.
- Liu, R.; Novak, J.; Hilscherova, K. In Vitro Assessment of Thyroid Peroxidase Inhibition by Chemical Exposure: Comparison of Cell Models and Detection Methods. Arch Toxicol. 2024, 98, 2631–2645.
- Larson, C.A.; Dashwood, R.H.; Bisson, W.H. Tea catechins as inhibitors of receptor tyrosine kinases: Mechanistic insights and human relevance. Pharmacol Res. 2010, 62(6), 457–464.
- Dong, H.; Godlewska, M.; Wade, M.G. A rapid assay of human thyroid peroxidase activity. Toxicol. In Vitro. 2020, 62, 104662.
