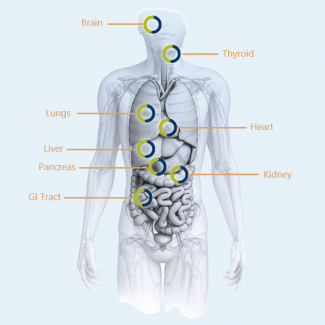
Human Biospecimens
LifeNet Health LifeSciences Human Biospecimens for research supports the advancement of science and medicine by providing researchers with high-quality samples from non-diseased control and disease specific deceased donors. LifeNet Health adheres to the highest operational and ethical standards to provide safe and reliable solutions in a variety of tissue types and preservation formats from fully authorized donors whose gifts were not suitable for clinical transplantation.
With 40 years of experience in tissue recovery and processing, our trained staff collaborate with each researcher to create a research tissue protocol that maximizes tissue quality. These resources help fulfill unmet needs across pharmaceutical, biotech, academic, and government sectors while honoring the donors’ desire to improve lives through scientific discovery.
- Industry-leading recovery, transport, and handling protocols ensure tissue integrity is consistently maintained and adheres to the highest standards
- Comprehensive donor medical and social history provided with full authorization and research disclosure
- Recovery of tissues with low post-mortem interval (PMI) from 1-24 hours
- Broad range of donated tissue recovery capabilities
- Direct access to technical expertise and guidance from LifeSciences’ team of scientists
- 24/7 recovery and logistics with the ability to handle international shipping and short delivery times
- Negative or non-reactive test results for HIV-I/II, HBV, HCV (including NAT testing), RPR/STS
- Custom sample collection methodologies available
- Preservation formats include:
- Fresh samples
- Formalin fixed
- Frozen
Human Biospecimens are suitable for multiple applications, including:
- Cell isolation
- 2D and 3D cell culture
- Genomics
- Proteomics
- Organotypic culture models – Co-cultures, 3D models
- Drug discovery and development
- Disease progression research
- Biomarker discovery
- Normal and high through put cell assays
- Exploration studies
- Non-diseased control tissue
Biospecimen Recovery Capabilities
LifeNet Health LifeSciences is the Direct Primary Tissue Source
LifeNet Health’s vertically integrated infrastructure allows us the ability to directly provide research organs and tissues for many types of research projects. LifeNet Health LifeSciences is the only biospecimens provider in the world that spans the donation continuum — from ethical donor consent to protocol-based recovery by highly trained personnel, to tightly controlled transport logistics designed to minimize ischemia time.
Our professional staff work carefully with each researcher to generate a research tissue protocol designed to maximize the research tissue quality. Researchers work directly with our screening and procurement teams to build specific project guidelines for donor selection, recovery methods, and preservation.
Begin building your project through our Biospecimen Form or reach out to one of our team members to discuss your needs.
Ethical Authorization for Research Donation
LifeNet Health adheres to the highest standards of ethical, safe research recovery, prioritizing the comfort and respect of the donor and donor family. Our authorization for research donation includes:
- Specific tissue authorization, including permission for genetic testing and storage for future applications.
- Full disclosures for board use of biospecimens and cells
- Authorization conducted by trained, professional transplant donation personnel
- Practices developed in conjunction with an Ethical Legal Social Implications study and in compliance with the Uniform Anatomical Gift Act
The Features of LifeNet Health Prospective Research Recoveries
- Specific protocols designed around donor selection, recovery techniques, preservation methods, and delivery options
- Exact instructions utilized to ensure recovered biospecimen meet research and experiment requirements
- Organ and tissue collection within specific age groups and with the presence or absence of disease and/or treatment
- Fresh tissue and organs available in addition to other preservation methodologies
The Importance of Utilizing Human Organs and Tissue for Research
Translational research is necessary to quickly go from bench to bedside and lead to groundbreaking discoveries. Utilizing human organs and tissues for research can provide researchers with relevant human data and responses for a range of applications including, but not limited to, predictive drug/chemical models, investigational drug studies, and the evaluation of disease progression and toxicity response research.
The use of primary human cells and tissues in translational research has become a federally approved alternative to animal trials in many cases. While this reduces the financial risk for many organizations, the true impact comes from the genetic diversity introduced using these cells and tissues. While cell culture and proliferation may be simple for cell lines, these models lack genetic diversity, leading to either low efficacy or health hazards at time of clinical trials1,2. Many animal models suffer the same outcome3,4. With the introduction of the FDA Modernization Act 2.0 in 2025, human cell-based assays and utilization of human biospecimens are now permitted in place of animal testing, allowing for a more cost-effective way to test a wide array of biological technologies in a way that accounts for population diversity.
[1] J. Puleo and K. Polyak, ‘The MCF10 Model of Breast Tumor Progression’, Cancer Res, vol. 81, no. 16, pp. 4183–4185, Aug. 2021, doi: 10.1158/0008-5472.CAN-21-1939.
[2] A. M. Rahimi, M. Cai, and S. Hoyer-Fender, ‘Heterogeneity of the NIH3T3 Fibroblast Cell Line.’, Cells, vol. 11, no. 17, Aug. 2022, doi: 10.3390/cells11172677.
[3] G. Suntharalingam et al., ‘Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412.’, N Engl J Med, vol. 355, no. 10, pp. 1018–28, Sep. 2006, doi: 10.1056/NEJMoa063842.
[4] P.-J. H. Zushin, S. Mukherjee, and J. C. Wu, ‘FDA Modernization Act 2.0: transitioning beyond animal models with human cells, organoids, and AI/ML-based approaches.’, J Clin Invest, vol. 133, no. 21, Nov. 2023, doi: 10.1172/JCI175824.
[5] FDA Modernization Act 2.0. 117th Congress (2021-2022), 2022. Accessed: Feb. 01, 2024. [Online]. Available: https://www.congress.gov/bill/117th-congress/senate-bill/5002






