Endocrine Disruption Assay Services
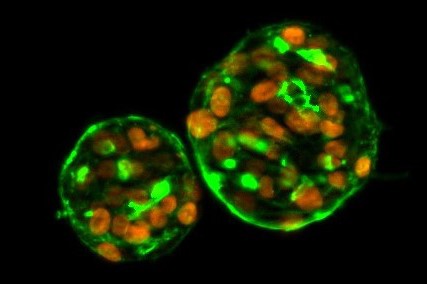
LifeNet Health offers a complete suite of assays to evaluate potential disruption of the endocrine system, including our 3D Thyroid Microtissue model for TDCs. Analyzing potential disruptions in vitro ensures accurate prediction of the effects of compounds on the endocrine system of target species in vivo. Our team of expert scientists can help in identifying the relevant endpoints to measure and the appropriate cells, and culture conditions, to accurately determine effects on the endocrine system
Connect with an expert
Tests We Offer
- Estrogen Receptor Binding
- Estrogen Transactivation (OECD 455)
- Androgen Receptor Binding
- Androgen Transactivation
- Aromatase Inhibition
- H295R Steroidogenesis Assay (OECD 456) with a Comprehensive 13 Steroid Panel
- Thyroid Hormone Disruption Assay - Direct Disruption
- Thyroid Hormone Disruption Assay - Indirect Disruption
Estrogen and Androgen Receptor Binding Assays
Understanding receptor binding is only part of the story. Receptor binding followed by a functional result of that binding provides a more complete picture. Agonist as well as antagonist activity can be determined with these assays, which use cell lines transfected with a luciferase reporter gene downstream from an ER or AR response element. The assay is called ER/AR transactivation also known as ERTA and ARTA. These assays are also provided by LifeNet Health and follow OECD 455 test guideline for ERTA.
Estrogen and Androgen Transactivation Assays
Several chemicals and drugs have been shown to interact with the endocrine receptors or interfere with hormone metabolism and function. This can result in non-genomic effects that include induction or inhibition of key cellular processes that control cell proliferation, fetal development, and reproductive function. Identification of these substances is critical to ensure that exposure does not result in adverse health effects related to endocrine disruption. Estrogen (ER) and androgen (AR) receptor binding is a first-tier assay. This is a competitive binding assay that utilizes human full length recombinant receptors. Receptor binding alone is only one part of an endocrine interaction. The ability to bind ER or AR and then elicit a downstream effect provides functional data.
Aromatase (CYP19) Inhibition Assay
The enzyme known as aromatase or (CYP19) is responsible for converting androgens to estrogens during steroidogenesis. The enzyme is vitally important for normal reproductive development in both males and females. The assay uses recombinant human CYP19 microsomes to determine aromatase inhibition. The assay can provide an IC50 which allows the relative potency of known and unknown aromatase inhibitor chemicals to be compared.
Steroidogenesis Assay
Steroidogenesis is a complex highly regulated pathway that begins with cholesterol and ends with the formation of estradiol and testosterone. The assay allows for the testing of chemicals that may disrupt the production of estrogen (E2) and testosterone (T). The assay utilized a human adeno-carcinoma cell line known as H295R cells. The assay measures changes in the production of E2 and T following administration of the test chemicals. LifeNet Health follows OECD 456 test guidelines when conducting this testing. In addition to the two end products (E2/T) many of the intermediate products can also be measured, if requested.
Identifying Thyroid Disrupting Drugs or Chemicals
LifeNet Health is pleased to provide assessments of thyroid hormone disruption using a human 3D thyrocyte model developed in collaboration with USEPA. The thyrocytes recapitulate the structure and function of the thyroid gland when they form a 3D microtissue. The cells in culture remain viable for several days which enables longer-term studies. Endpoints for this model include secretion of thyroxine (T4), Thyroglobulin (TG), and thyroperoxidase activity (TPO). The cells respond to the administration of thyroid stimulating hormone (TSH) by releasing T4. The cells can be used in our microphysiological system platform to include thyroid-hormone events associated with liver metabolism which may lead to pathologies such as goiter and thyroid tumors.
Our Process Sets Us Apart From Other Laboratories
Consultation – We partner with you to determine the best approach, offering customized study plans that draw from our 60-plus years of combined in vitro testing experience. We may recommend utilizing more than one assay when performing a thorough cytotoxicity screening.
Assay Development – We develop and validate your assay using many different cell models, with an emphasis on all-human primary cells and biospecimens to simulate in vitro conditions, which provides more relevant, reliable results than traditional testing methods. This is part of our commitment to advancing alternative methods to support organizations in moving away from traditional animal-based models.
Data Analysis – We provide expert data analysis, including offering adaptable options when needed based on early results.
Results Review – Our clear, concise reports bring simplicity to your biggest data challenges, putting your organization in the best position for regulatory reviews. We may suggest follow-up studies to further your organization’s research goals.
Our facilities are compliant with Good Laboratory Practices and Good In Vitro Method Practices (OECD 286).
