J. Pregenzer1, D. Austin1, J.M. McKim1
1LifeNet Health LifeSciences, Kalamazoo, MI
Abstract
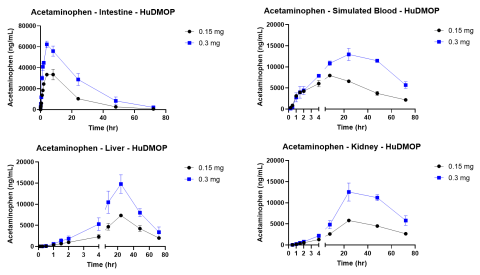
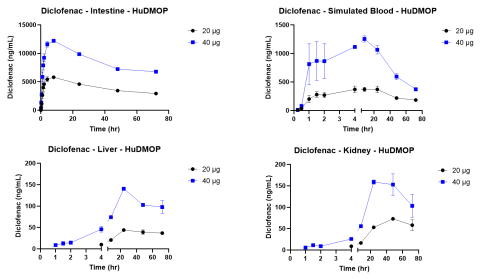
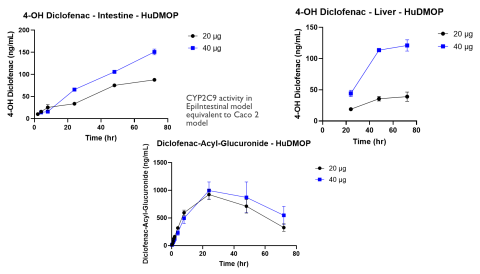
Since the publication of the National Science Foundation’s “Toxicity Testing in the 21st Century,” considerable progress has been made in the sophistication of human and animal cell and tissue models and the technology platforms that incorporate them. The introduction of new approach methodologies (NAMs) is changing how in vitro toxicology assessments are done, and the concept of Next Generation Risk Assessment (NGRA) provides a way to utilize the datasets generated by these approaches. One technology that holds promise is microphysiological systems (MPS). These platforms incorporate fluidics and cell culture on a micro scale and have led to the organ-on-a-chip concept. To be useful these MPS models must allow the linking of multiple organ models and should simulate in vivo barriers. The complete system should also provide concentration vs. time curves for drugs or chemicals and identify metabolites and cytotoxicity. The primary aim of this study was to evaluate two known drugs, acetaminophen and diclofenac, in a new meso-scale MPS that integrates multiple organs via a simulated blood flow system. The in vitro MPS that was used consisted of a three-compartment circuit (intestine, liver, and kidney). The human 3D EpiIntestinalTM tissue was obtained from MatTek Corp.; primary human hepatocytes from LifeNet Health were used for the liver; and the kidney was modeled with a normal human renal proximal tubule cell line (HK-2). Each organ compartment was isolated with no fluid exchange between organ compartments. This allowed each organ model to be cultured under its own optimal conditions. Communication between organs was by a simulated blood flow system that incorporates a semipermeable membrane inside each organ compartment. This allowed the test drugs and their metabolites to move by osmotic diffusion into and out of the simulated blood flow and organ compartments. Salts and other growth medium components are osmotically balanced. Flow was accomplished with a precision micro syringe pump (5 µL/min) and the simulated blood consisted of buffered saline (pH 7.4) with human serum albumin (0.1-0.4%). APAP or diclofenac were applied to the apical surface of the intestine chamber (0.15 and 0.3 mg for APAP and 20 and 40 µg for Diclofenac) in a volume of 100 µL. Samples (50 µL) were collected from each compartment at 0 hr, 5 min, 15 min, 30 min, 60 min, 1.5 hr, 2 hr, 4 hr, 8 hr, 24 hr, 48 hr, and 72 hr. These samples were analyzed by LC/MS/MS for APAP or diclofenac, with the data used to generate concentration vs. time curves. These kinetic curves showed clear absorption and elimination phases. The glucuronide conjugate of APAP and the 4-OH and acyl-glucuronide metabolites of diclofenac were also measured. Following the 72 hr collection time the tissues were harvested and used to measure cytotoxicity. The primary organ for toxicity was the liver with APAP causing a 40-60% loss in ATP, while cell integrity remained high. Diclofenac also reduced ATP in a dose-dependent manner. These findings indicate mitochondrial mediated hepatotoxicity, which is consistent with in vivo reports. By combining kinetic data, chemical properties, and cytotoxicity data it will be possible to develop quantitative models for predicting exposure and identifying hazard for NGRA.
Introduction
The use of in vitro systems to evaluate the safety and pharmacokinetics of new drugs requires the ability to link multiple organs together with a flow or simulated blood system. Although there have been many new organ-on-a-chip models in recent years, these systems while having strong bioengineering components lack some key features of biology. These include normal barriers to diffusion and basic biological compartments such as intracellular, extracellular and intravascular. Cells and tissues that are used to mimic in vivo organs must be well characterized and possess the biological functions that define and characterize normal organ function. When each in vitro organ system is well characterized with regard to metabolic function, drug metabolizing enzymes and uptake/efflux transporters it should be possible to develop in vitro pharmacokinetic data sets that allow models to be built to extrapolate in vitro data to in vivo exposure, metabolism, and safety. The human 3D EpiIntestinal model developed by MatTek Corp. is well characterized in terms of tissue morphology, drug metabolizing enzymes, and transporters (Cui et al. (2020; Markus et al. 2021). The primary human hepatocytes provided by LifeNet Health are well characterized in terms of plateability, longevity in culture, morphology, genotype, metabolizing enzymes and transporter function and orientation. For the kidney compartment normal human renal proximal tubule cells were used (HK-2) cell line (Garcia-Perez et al. 2021; Qiu et al. 2018). When combined with a simulated blood flow system this tri-organ system provides in vitro data sets that can be compared with known in vivo information. This enables the development of mathematical models that extrapolate the in vitro data to in vivo effects and exposures. Once validated, this type of in vitro system can be used to rapidly understand basic DMPK parameters as well as issues related to target and off-target toxicity. These data can then be used in next generation risk assessment approaches (NGRA) (Jagiello and Ciura, 2022;Li et al., 2021).
Materials & Methods
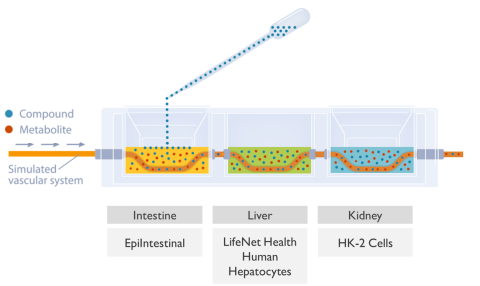
The intestine was modeled using EpiIntestinal by MatTek Corp.. The liver compartment was modeled with well-characterized primary human hepatocytes from LifeNet Health, and the kidney chamber was normal human renal proximal tubule cells (HK-2) from ATCC. The three organ systems were completely isolated and cultured with media specific for each tissues optimal growth. Each tissue chamber held 2.5 mL of medium. Communication between compartments was achieved by a simulated vascular system driven by a micro syringe pump at 5 µL/min. The section of vascular tubing inside each compartment was a semipermeable membrane (Mw cut off 30K da.). The movement of test material from one compartment to another is by osmotic movement of the test material (APAP and Diclofenac) and any metabolites into and out of the simulated vasculature system. The fluid being pumped through this tubing can be modified as needed, but typically consisted of phosphate buffered saline (PBS pH 7.4) and 0.1% human serum albumin (Figure 1).
The kinetic experiments were started by adding 100 µL of the APAP (150 or 300 mg/mL) or Diclofenac (200 or 400 µg/mL) stock solutions to the apical side of the EpiIntestinal model. Samples from all compartments were collected at the indicated times and the amount of UA determined by LC/MS/MS. Following the 72 hr exposure the tissues were collected and analyzed for cell health. Assays included the (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay for the intestine tissue, adenosine triphosphate (ATP) and glutathione (GSH) for the liver tissue, and N-acetyl-β-d-glucosaminidase (NAG) for the kidney.
Results

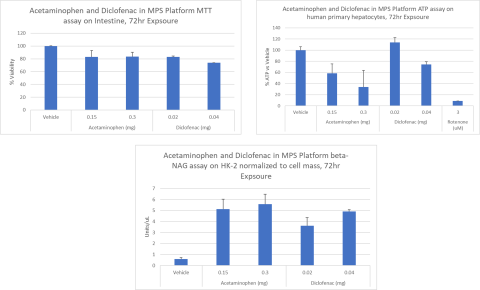
Conclusions
The MPS platform described in this study provided dose dependent kinetic, metabolism, and toxicity data that was consistent with published literature for both test compounds.
These data will assist in the development of mathematical models designed to extrapolate the in vitro information to in vivo exposure and hazard identification.
This type of MPS platform is versatile and can be used with many cell and tissue models already validated. The system allows collection of parent compound and metabolite kinetic data and provides the basis for NGRA and IVIVE models.
References
Cui Y. et al. (2020) In-depth characterization of epiintestinal microtissue as a model for intestinal drug absorption and metabolism in human. Pharmaceutics 12, 405.
Garcia-Perez E. et al. (2021) Human proximal tubule epithelial cells (HK-2) as a sensitive in vitro system for Ochratoxin A induced oxidative stress. Toxins 13, 787.
Markus J. et al. (2020) Human small intestinal organotypic culture model for drug permeation, inflammation, and toxicity assays. In vitro Cellular Devel Biol 57, 160-173.
Xuan Qiu et al. (2018) An in vitro method for nephrotoxicity evaluation using HK-2 human kidney epithelial cells combined with biomarkers of nephrotoxicity. Toxicol Res 7, 1205-1213.
