A.T. Salminen, R. Gillespie, B. Brame, J.M. McKim, Jr.
LifeNet Health LifeSciences, Virginia Beach, VA
Abstract
Background and Purpose: In vitro permeation testing is a critical tool in the evaluation of chemical absorption through the skin, providing essential data for the development and safety assessment of topical formulations. The OECD Test Guideline 428 (OECD 428) as well as opinion SCCS/1358/10 outline standardized criteria for conducting in vitro permeation tests to ensure consistency and reliability across studies. Building on these guidelines, including developing reliable frameworks for the procurement of human skin samples, is necessary for continued success in the execution of such assays.
Methods: Excised human skin (EHS) from the upper leg and back was recovered within the LifeNet Health tissue procurement network, made possible by the generous gift of an individual or their family. Minimal (<24h) warm ischemic time criteria was implemented to ensure skin integrity was maintained. EHS was dermatomed to a uniform thickness of 500 μm in line with regulatory guidance, and subsequently preserved at -80 °C for long-term storage. Processed EHS was qualified for in vitro permeation testing by mounting on Franz diffusion cells (0.64 cm2 active area) and quantifying transepidermal water loss (TEWL) as well as 24h permeation of an infinite dose (250 μL/0.64 cm2) of OECD-reference compound, caffeine (1% w/v in aqueous buffer). Caffeine present in the receptor chamber of the Franz diffusion cell was quantified at 0, 0.5, 1, 2, 3, 4, 6, and 24 hours post-dosing via liquid chromatography tandem mass spectrometry (LC-MS/MS). Additionally, a wash of the apical skin surface was performed at 24h post-dosing, and wash fluid, along with the extracted tissue, was analyzed for caffeine by LC-MS/MS.
Results: Dermatomed EHS from both anatomical regions, back and leg, showed consistent TEWL (<15 g/m2 h) suggestive of conserved skin integrity. Further, under a regulation-compliant and optimized protocol, 24h-permeation of the OECD-reference compound, caffeine, was in line with literature report values (between 1-4% of dose applied). Additionally, the 24h mass balance (wash fluid, tissue, receptor fluid) was consistent and within regulatory requirements of 100% ± 15% of dose applied.
Conclusions: Dermal absorption data is necessary for predicting potential systemic exposure of new test compounds following topical application. Consistent interpretation and implementation of regulatory guidance is necessary to obtain reliable in vitro permeation testing data. Expanded skin procurement guidance is an underlying gap in these guidelines with the utmost importance to the execution of this assay. Skin procurement methodology demonstrated herein permitted successful and reproducible execution of a regulatory-compliant in vitro permeation test.
Introduction
In vitro permeation testing (IVPT) is a method used to evaluate the permeation characteristics of topical drug products, cosmetic ingredients, and environmental exposures through the skin (Salminen et al., 2023). This assay can be used alongside in vivo methods to support regulatory submissions for topical drugs, such as in FDA’s Abbreviated New Drug Applications (ANDAs) for bioequivalence demonstrations. For cosmetic ingredients and environmental chemicals, IVPT provides crucial data for risk assessments to ensure consumer and public safety. The Organization for Economic Co-operation and Development (OECD) has established Test Guideline 428 (OECD 428), which outlines in vitro methods for skin absorption studies. It specifies criteria for essential testing materials, including diffusion cells, receptor fluid, and skin. Additionally, it details experimental parameters such as test article preparation and dosing, skin temperature and integrity, and experimental timing and sample collection. Finally, it provides guidelines for sample analysis and data reporting. The European Commission’s Scientific Committee on Consumer Safety (SCCS) opinion SCCS/1358/10, along with OECD’s guidance notes on dermal absorption studies (OECD No. 156), further clarify and build on OECD 428 to support consistent execution of IVPT across laboratories.
Materials & Methods
Materials
Test Article: Caffeine (CAS-No. 58-08-2) purchased from Sigma (Cat. # C0750-5G) and formulated in 1X DPBS at 1% w/v (10 mg/mL).
Background Control: 1X Dulbecco’s Phosphate-Buffered Saline (1X DPBS) with calcium and magnesium purchased from Corning (Cat. # 21-030-CM).
Extraction Fluid: 50% v/v methanol in Ultrapure water was prepared using Methanol anhydrous, 99.8% purchased from Sigma (Cat. # 322415) and Ultrapure Water purchased from ThermoFisher (Cat. # 10977023).
Receptor Fluid: 1X Dulbecco’s Phosphate-Buffered Saline (1X DPBS) with calcium and magnesium was purchased from Corning (Cat. # 21-030-CM).
Wash Fluid: 200 Proof Ethanol was purchased from Decon Labs (Cat. # 3916).
IVPT Methodology
The in vitro permeation test was performed in accordance with OECD Test Guideline 428. Franz-cell (PermeGear, Cat. # 4G-01-00-09-05) mounted skin temperature (32 ± 1°C) was verified after equilibration of the system, prior to dosing, with an infrared thermometer (VWR, Cat. # 36934-180). To confirm skin integrity, transepidermal water loss (TEWL) was measured across Franz cell-mounted skin with a VapoMeter (Delfin Technologies), immediately prior to dosing.
All test compounds remained on the skin for the duration of the experiment (24-hour exposure). Receptor fluid collections were made at 0-, 0.5-, 1-, 2-, 3-, 4-, 6-, and 24-hours post-application of the test article or control. Following experimentation, the apical skin surface was washed to remove un-absorbed test article. Wash fluid was collected with a cotton pad. Cotton pads (wash) and tissues were extracted in 50% v/v methanol in UltraPure water (extraction fluid).
Bioanalytical Testing
Bioanalytical testing to quantify caffeine in the receptor fluid collections and wash and tissue extracts was performed using a Shimazdu Nexera XR LC-30AD UPLC system in-line with a SCIEX 6500+ mass spectrometer via an electrospray ionization (ESI) interface (i.e. LC-MS/MS) with a 1 ng/mL limit of quantitation.
Data Analysis
The cumulative amount of analyte in the receptor fluid was calculated from the LC-MS/MS results for each collection (n) as follows:
When 𝑛𝑛 = 1, Cumulative Amount = (RFn)Vr
When 𝑛𝑛 > 1, Cumulative Amount = (RFn)VR + (∑n-1i=1 RFi x Vs)
Where RFn is the concentration of analyte in receptor fluid sample (n), VR is the total volume of the receptor compartment (5 mL), and VS is the volume of the sample (500 μL). Sample n = 1, 2, 3, 4, 5, 6, 7, 8 corresponds to receptor fluid collections at 0, 0.5, 1, 2, 3, 4, 6, and 24 hours post-dosing, respectively. Cumulative amount was normalized to the surface area of the Franz diffusion cell orifice (0.64 cm2) and reported as ng/cm2.
Flux, reported as ng/cm2/h, was calculated by dividing the surface area-normalized amount of analyte permeated between collection times by the time between collections.
Mass distribution of the analyte was calculated for each compartment, wash, tissue and receptor, by dividing the total amount of analyte in each compartment at 24 hours post-dosing by the amount of analyte dosed.
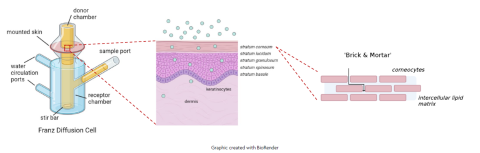
A graphic representation of the principles of the in vitro permeation test. The dermal absorption of a test article may be evaluated in vitro, through excised skin mounted on a diffusion cell (e.g. static Franz diffusion cell). The lipid-rich stratum corneum provides a selective barrier to permeation and, together with the viable epidermis, regulates systemic exposure of topically applied compounds.
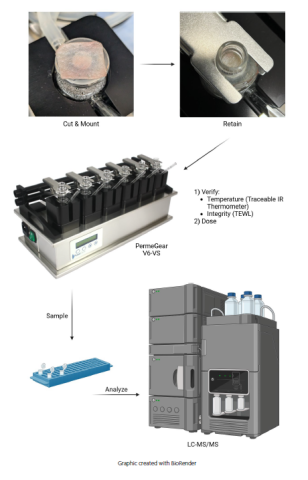
Fig. 2. An OECD 428-compliant experimental approach to in vitro permeation testing. Excised human skin (<1 mm thick) is cut and mounted on the receptor chamber of the static Franz diffusion cell (minimum active surface area of 0.64 cm2) and retained with the donor chamber and retaining clip. Mounted skin temperature (32 ± 1°C) and integrity (lab-specified) are verified using an IR thermometer and trans-epidermal water loss (TEWL) meter, respectively. Skin integrity may alternatively be assessed by trans-epidermal electrical resistance (TEER) or tritiated-water absorption. Once the test system is established, the test article is applied to the apical skin surface (infinite or finite dose [1-5 mg/cm2 or 10 μL/cm2]) and receptor fluid samples are collected over the experimental window (e.g. 24 hours). A minimum of four replicates per test preparation are performed in parallel with a reference control and background control. Receptor fluid, wash, and tissue samples are extracted (when appropriate) and analyzed, and overall recoveries and absorption profiles are reported.
Results
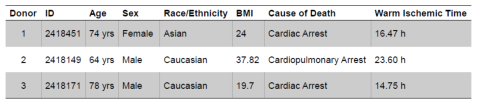
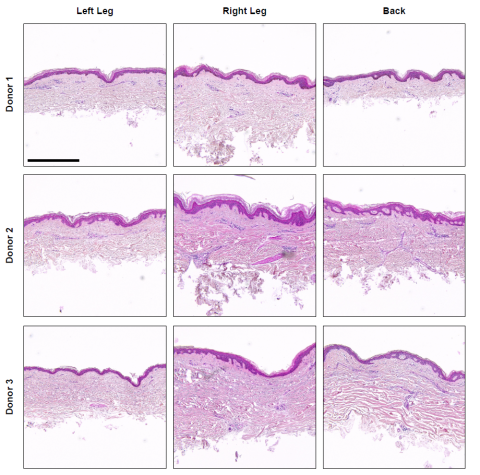
Fig. 3. Histological assessment of dermatomed excised human skin. Skin tissue was recovered from the left and right circumferential upper leg or back of three donors (Table 1) within the LifeNet Health tissue procurement network. Skin tissue was processed with a rotary dermatome (Exsurco), with a setpoint thickness of 500 μm to isolate the epidermis and partial dermis, and placed between slip sheets, packaged, sealed, and frozen (-80°C). Excised human skin samples from each donor and anatomical region were processed for histological assessment. Briefly, samples were fixed in 10% neutral buffered formalin and processed and vacuum infiltrated with paraffin on a Sakura VIP 2000 tissue processor; followed by embedding with a ThermoFisher HistoCentre III. Embedded samples were placed on a Reichert Jung 2030 rotary microtome, faced to expose tissue sample, and finely sectioned at 4-5 microns. Slide-mounted sections were stained on a Leica Autostainer XL with a routine Hematoxylin and Eosin method followed by coverslipping with synthetic mounting media for permanent retention and visualization with light microscopy. Representative micrographs were collected on a Cytation5 Cell Imaging Multi-Mode Reader (Agilent BioTek) with a 10X objective. Scale bar = 500 μm.
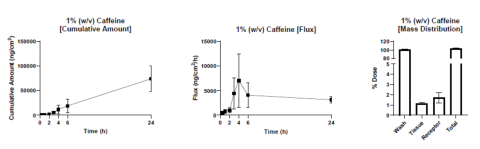
Fig. 4. In vitro permeation testing proficiency data collection – caffeine. Dermal absorption of an infinite dose (250 μL/0.64 cm2) of caffeine (1% w/v in 1X DPBS) was assessed through excised human skin. Receptor fluid collections were performed at 0, 0.5, 1, 2, 3, 4, 6, and 24 hours post-application of the test article. At 24-hours post-dosing, the apical skin surface was washed, and the skin was collected for independent analyses. The caffeine amount in each sample was quantified by LC-MS/MS. Cumulative amount (left), flux (middle), and mass distribution (right) were calculated from LC-MS/MS results. N = 10 independent Franz cells and three individual skin donors. Mean ± standard error of the mean are presented. All skin sections demonstrated a TEWL less than 15 g/m2 h.
Conclusions
- Consistent interpretation and implementation of regulatory guidance on in vitro permeation testing is critical for accurate risk assessment.
- IVPT methodology presented herein provide reproducible and traceable results under a range of test article scenarios.
- Minimized (<24 hours) warm ischemic time and consistent excised skin procurement and processing preserve skin barrier integrity for dermal absorption assessments.
- Multiple anatomical regions from a single donor may be tested in parallel under these procurement processes, allowing for expanded compound evaluation.
References/Acknowledgements
OECD. (2004). Test No. 428: Skin Absorption: In Vitro Method. https://doi.org/10.1787/9789264071087-en
OECD. (2022). Guidance notes on dermal absorption studies, second edition. Series on Testing and Assessment, No. 156. ENV/JM/MONO(2011)36/REV1; OECD Homepage.
Salminen, A. T., Davis, K. J., Felton, R. P., Nischal, N., VonTungeln, L. S., Beland, F. A., Derr, K., Brown, P. C., Ferrer, M., Katz, L. M., Kleinstreuer, N. C., Leshin, J., Manga, P., Sadrieh, N., Xia, M., Fitzpatrick, S. C., & Camacho, L. (2023). Parallel evaluation of alternative skin barrier models and excised human skin for dermal absorption studies in vitro. Toxicol In Vitro, 91, 105630. https://doi.org/10.1016/j.tiv.2023.105630
SCCS. (2010). Basic criteria for the in vitro assessment of dermal absorption of cosmetic ingredients. https://doi.org/10.2772/25843
The authors would like to acknowledge the Michigan State University HistoPath Lab for their support of the histology work presented herein. We also wish to express our deepest gratitude to the donors and their loved ones for saying 'yes' to donation. This selfless act has enabled our pursuit of enhanced safety and risk assessment of dermatological products that consumers and patients rely on every day, and we are truly grateful.
