Stephanie Piekos1, Jessica Weaver2, Cody Thomas1, Alexander Byer-Alcorace1, Justin J. Odanga2, Kristina K. Wolf3, Jingsong Chen2, Jung Bok Lee3, Edward L. LeCluyse3, and Mitchell E. Taub1
1Non-clinical DMPK, Boehringer Ingelheim Pharmaceuticals Inc., Ridgefield, CT; 2Institute of Regenerative Medicine, LifeNet Health, Virginia Beach, VA; 3Research and Development, LifeSciences Division, LifeNet Health, Research Triangle Park, NC
Abstract
Routine use of primary human hepatocytes (PHH) for clearance estimations of low-turnover compounds has been restricted in part by the lack of suitable technology that is convenient, supports a multitude of donor lots, and maintains the metabolic pathways of PHHs over prolonged culture periods. To address these limitations, an all-human triculture system has been developed comprised of cryopreserved PHHs and primary feeder cells (FCs). FCs are stromal and endothelial cells at a 1:1 ratio. Frozen FCs were thawed and seeded onto 24-well collagen coated plates and allowed to attach. Cryopreserved adult PHHs were then thawed and plated onto the FCs, creating a Triculture System (TCS). Basic morphology and functionality, including formation of functional bile canaliculi, albumin (Alb) synthesis, and urea production were evaluated over a 4-week culture period. Cytochrome P450 (CYP), UDP-glucuronosyltransferase (UGT) and aldehyde oxidase (AO) activities were also determined over a 2-4 week period. The ability of the TCS to predict in vivo non-renal clearance of two slowly cleared compounds was also evaluated. PHHs from multiple adult donor lots in the TCS exhibited a healthy and stable morphology, including multicellular cluster formation, for up to 30 days in the 24-well format. Extensive anastomosing networks of bile canaliculi were identified with CDFDA staining after 5 days in culture with well-formed tight and gap junctions that remained stable throughout the rest of the culture period. Albumin and urea production levels were maintained in the TCS over a 4-week period. CYP1A2, CYP2C9, CYP2D6, and CYP3A4 specific activities were maintained at stable levels over the culture period. E2-3G production (UGT1A1) was stable between day 5 and 28 of culture, while AZT-G formation (UGT2B7) was well-maintained but exhibited greater variability over the 4-week culture period. AO specific activity was greater in the TCS than in suspended PHHs and was stable over at least a 14-day period. The TCS was also able to predict the hepatic clearance of alprazolam and tolbutamide within 2-fold of reported in vivo values. Overall, these results show that the TCS represents a convenient, stable, all-human hepatic culture system that maintains both hepatocellular morphology and various metabolic functions for up to 1 month.
Objectives
1. Establish stable hepatocyte tricultures.
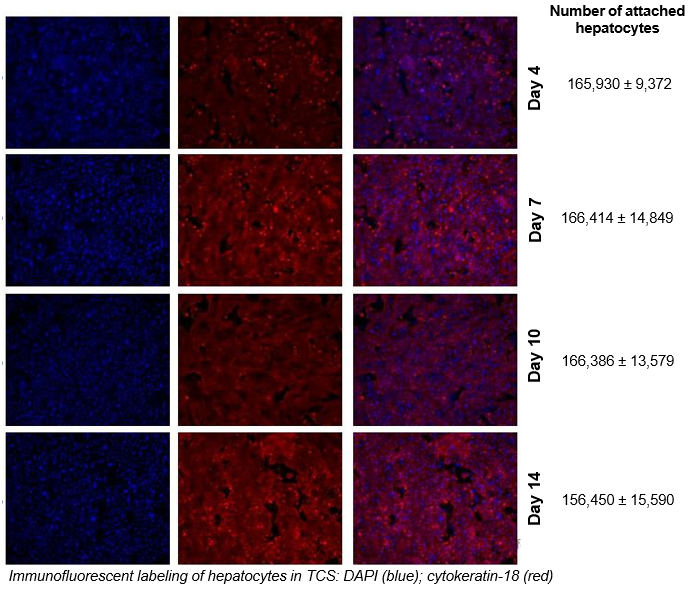
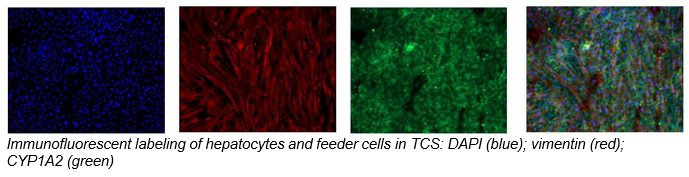
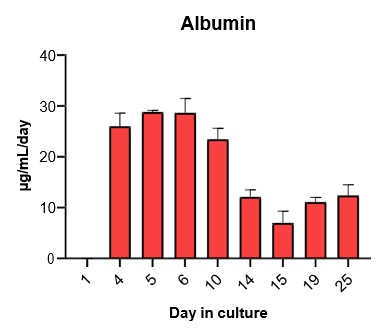
2. Characterize hepatocyte morphology and functionality over a 4-week culture period by assessing albumin and urea production, bile canaliculi formation, and CYP3A4 protein.
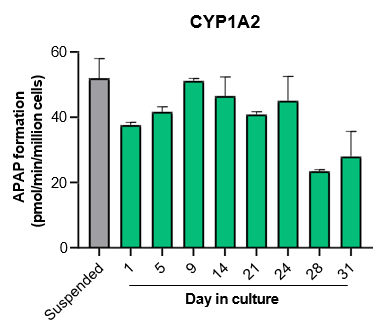
3. Assess activity of CYPs, UGTs, and AO over a 4-week culture period. Compare with suspended hepatocytes by measuring metabolism probe substrates.
4. Assess inducibility of major CYP enzymes in response to prototypical inducers by measuring gene expression.
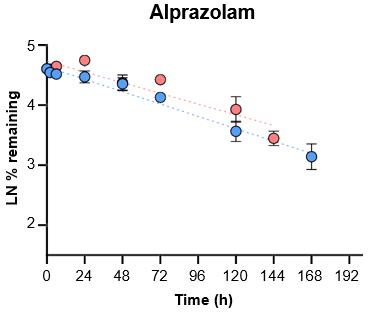
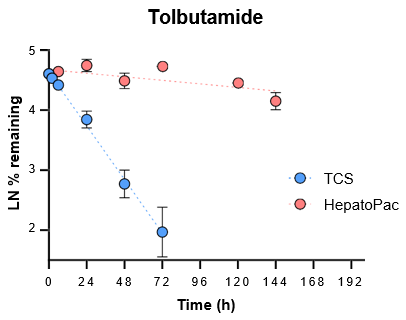
5. Evaluate the ability of the TCS to predict in vivo clearance of slowly metabolized compounds by incubating with tolbutamide and alprazolam for seven days.
Results
Hepatocytes in the TCS retain functionality for up to four weeks in culture.
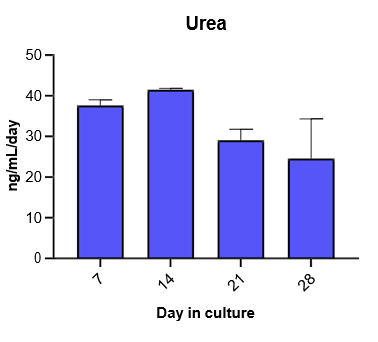
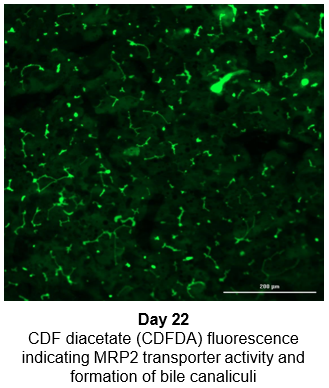
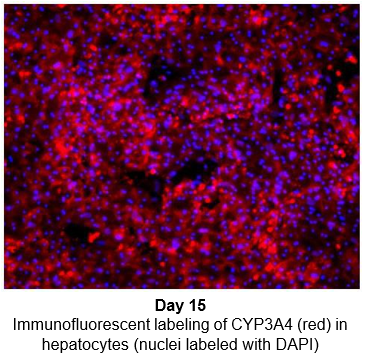
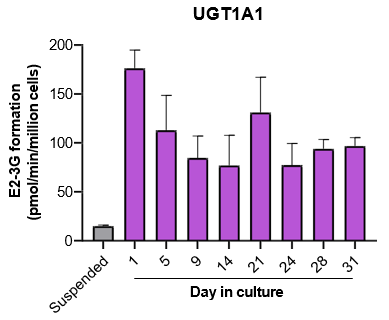
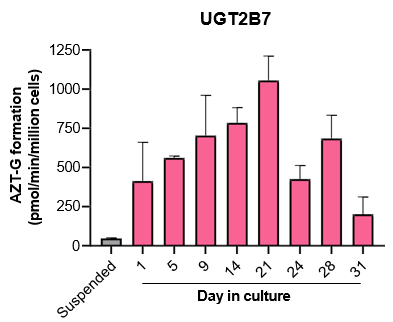
The activity of CYP enzymes, AO, and UGT enzymes persists in the TCS for up to four weeks.
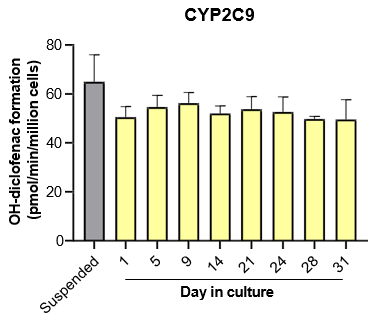
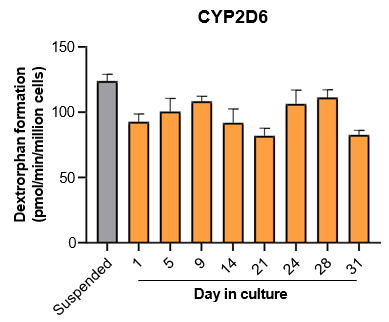
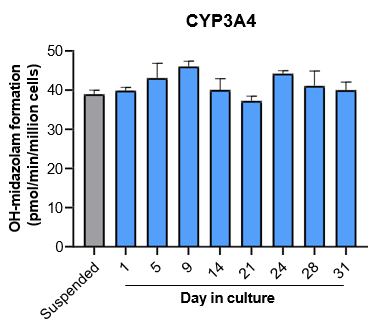
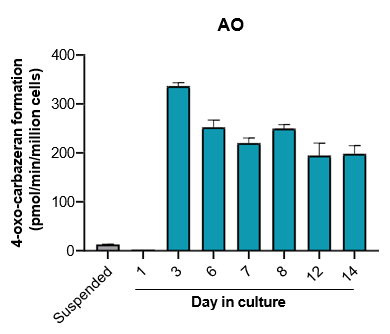
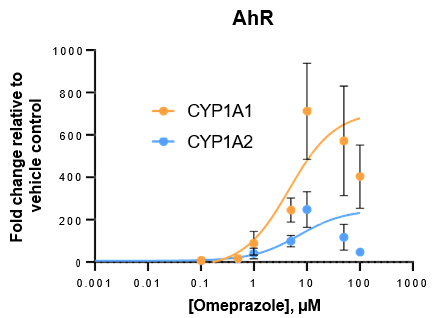
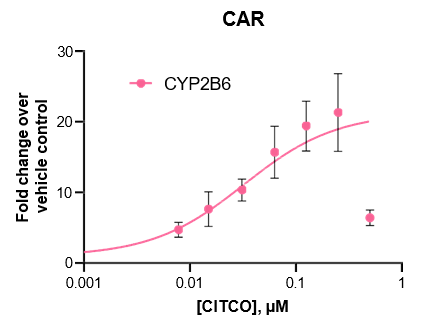
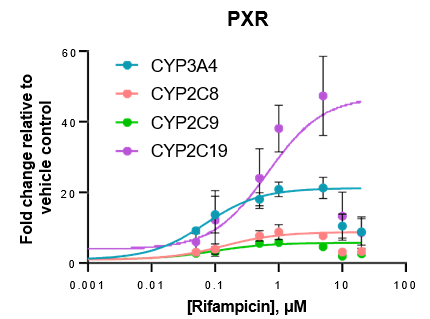
Robust induction of major CYP genes is observed following exposure to prototypical inducers
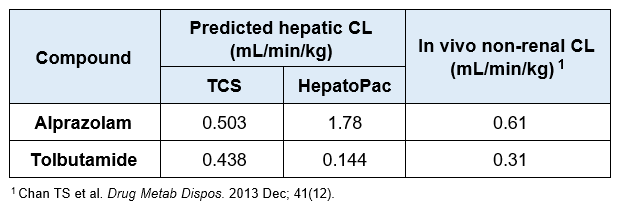
For slowly metabolized compounds, in vitro CLint generated in the TCS predicted in vivo non-renal CL within 2-fold.
Conclusions
- The TCS maintains hepatic function and high levels of drug metabolizing enzyme activity for up to four weeks in culture
- The activity levels of AO, UGT1A1, and UGT2B7 are significantly higher in TCS than in suspended hepatocytes from the same donor
- Expression levels of CYP enzymes in the TCS are highly inducible in response to prototypical inducers
- The TCS may be a useful in vitro model to predict in vivo clearance of slowly metabolized compounds
